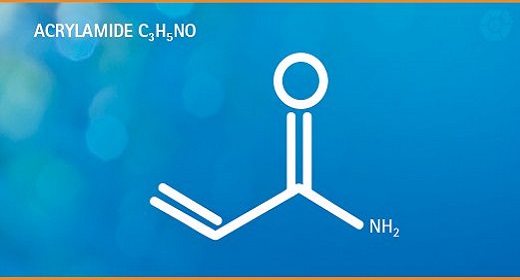
Acrylamide is a potentially toxic and potentially cancer-causing substance that can be naturally present in uncooked, raw foods in very small amounts…
But for this substance to pose a risk of toxicity or cancer, it must be present in foods in much larger amounts, and these larger amounts do not occur unless those foods have been cooked. In addition to certain cooked foods that can expose us to large amounts of acrylamide, there are many non-dietary sources exposure to this substance. These non-dietary sources include cigarette smoke (about 1-2 micrograms per cigarette) and cosmetics. There is also airborne release of acrylamide during many different manufacturing processes, including the manufacturing of paper, asphalt, petroleum, photographic film, construction adhesives, varnishes, and dyes. The U.S. Environmental Protection Agency (EPA) has estimated that U.S. adults average 0.4 micrograms of dietary acrylamide intake per kilogram of body weight each day. For an adult weighing 150 pounds, this amount translates into approximately 27 micrograms of dietary acrylamide per day.
EPA Guideline for Acrylamide Intake
In 2010, the EPA established an RfD (Reference Dose) for oral intake of acrylamide. Reference doses are amounts of daily exposure over a lifetime that can be predicted to produce no noticeable health effects. In other words, as a general rule, adverse health affects are not expected if daily intake of a substance remains below the reference dose level. The reference dose established for acrylamide by the EPA in 2010 was 0.002 milligrams of acrylamide per kilogram of body weight per day. For a person weighing 150 pounds, this RfD for acrylamide means a daily dietary exposure limit of about 140 micrograms. Since the EPA has estimated that an adult weighing 150 pounds averages about 27 micrograms of daily acrylamide intake from his or her diet, this RfD guideline suggests that on average, U.S. adults are getting about 19% of their maximum allowable exposure to acrylamide from dietary intake.
What Risks are Posed by Excessive Intake of Acrylamide?
Acrylamide is currently classified as a Group B2, probable human carcinogen. That EPA classification is primarily based on the fact that most of the acrylamide research has been conducted on animals, and large-scale epidemiologic studies on humans are simply not available. Acrylamide has also been shown to be a neurotoxin that can damage nervous system function. (It’s likely to accomplish this damage by disrupting the signal that gets sent by nitric oxide at the onset of the nerve firing process.) The neurotoxic and probable cancer-causing aspects of acrylamide make it clear that we do not want to expose ourselves to excess amounts of acrylamide from any source.
How are Large Amounts of Acrylamide Formed in Food?
In food, acrylamide can be formed in two basic ways. First, acrylamide can be formed when amino acids interact with sugars in the presence of heat. Many different kinds of sugars and many different amino acids can interact in this way. However, one particular amino acid—called asparagine—has a far greater tendency to interact with sugars and to form acrylamide than other amino acids.
Second, it is possible to form acrylamide without the presence of sugars. When fats in food are oxidized, unique 3-carbon molecules (including acrylic acid and acrolein) can be formed. In the presence of heat, these 3-carbon molecules can interact with asparagine to form acrylamide. It’s common for fried foods to form acrylamide in this way, even when there is little sugar found in the foods, no sugar added during frying, and little breakdown of starch into sugar.
One noteworthy example of acrylamide formation involves the conventional production of potato chips. There are small amounts of asparagine present in raw potatoes before processing. During the frying process, fats used for frying can be oxidized and can become converted into acrolein and acrylic acid. Starches in the potato can also be broken down into sugars. This unique mixture of substances can interact in a way that results in unusual amounts of acrylamide formation. Potato chips can commonly contain more than 1,000 parts per billion (ppb) of acrylamide. In a very small, one ounce snack-sized bag of potato chips, this amount would represent about 28 micrograms of acrylamide—an amount that is just above the average total exposure to acrylamide experienced by U.S. adults, and about 20% of the maximum safe intake of dietary acrylamide as established by the EPA.
In the above example, you’ll notice that potato chips can commonly contain more than 1,000 ppb. That’s the same as saying that potato chips reach up into the parts per million (ppm) range with respect to their acrylamide content. This high level is unusual. Most foods that contain acrylamide provide it in ppb levels, not ppm levels. Ppm levels are 1,000 times greater than ppb levels! When you consider the overall research on acrylamide in food, the list of foods potentially containing ppm levels is a very limited one.
What is the Relationship Between Acrylamide and Asparagine?
As described earlier, there is a far greater tendency for acrylamide to be formed from the amino acid asparagine than from any other commonly occurring amino acid. This fact might lead us to think that there would be a close relationship between the amount of asparagine found in food and its amount of acrylamide. Research has clearly shown that no such relationship exists. While it is important for asparagine to be present for the formation of acrylamide, there are too many other requirements that must be met before acrylamide can be formed. For this reason, the amino acid asparagine is simply a necessary—but not sufficient—factor when it comes to the formation of acrylamide.
As the table above indicates, foods must definitely contain at least minimal amounts of the amino acid asparagine in order for substantial amounts of acrylamide to be formed. However, the amount of acrylamide formed cannot be predicted based solely on the amount of asparagine found in a food. For example, the amount of asparagine found in asparagus can be relatively high, even though most studies show very low levels of acrylamide in this food. (The amino acid asparagine originally got its name because it was first detected in asparagus juice.)
How Does Heat and Cooking Methods Affect Acrylamide Formation?
There’s no question that heat and cooking are required for formation of unwanted amounts of dietary acrylamide. As evidenced by the chart above, heat and cooking do not always result in significant acrylamide formation, even if a food contains significant amounts of the amino acid asparagine. However, when all factors for forming acrylamide are present, it takes approximately 250°F/121°C for appreciable formation of acrylamide in most foods. Interestingly, acrylamide formation may peak in temperature ranges commonly used for roasting (250-375°F/121-191°C). We’ve seen research showing formation of acrylamide in green tea when roasted at these temperatures, and acrylamide formation in roasted coffee beans has also been shown to be substantial in this regard. The toasting of bread (also commonly done within this temperature range) has also been shown to increase acrylamide formation. A temperature range of 325-375°F/163-191°C is also frequently used for the frying in oil of french fried potatoes and potato chips. Once again, it is important to realize that the heating of foods at temperatures between 250F-375°F/121-191°C does not automatically mean that acrylamide is being formed in the food. It takes a combination of the amino acid asparagine together with a form of sugar, or the oxidation of fat into smaller carbon molecules, or both to result in substantial formation of acrylamide.
What About Acrylamide and Olives?
Research on olives and their acrylamide content has shown some inconsistency over the past several years and this inconsistency has sparked controversy in the public press about olives and their health risk with respect to acrylamide. In data assembled by the U.S. Food and Drug Administration (FDA), we’ve seen more than a dozen different kinds of olives, including Spanish, Greek, Kalamata, Nolellata, Sicilian, d’Abruzzo, and Gaeta, and di Cerignola that were determined to contain no detectable level of acrylamide. Yet we have also seen FDA data showing levels of acrylamide as high as 1,925 ppb in some canned, nationally distributed brands of black pitted olives. Based on this data, we suspect that these higher acrylamide levels in select canned black olives were related to specific handling, storage, processing (especially preservation and darkening methods), and heating steps that favored formation of acrylamide. It’s also important to note here that we are not aware of any data showing problematic levels of acrylamide in any extra virgin olive oils available in the marketplace.
At present, we are not aware of any foolproof method that consumers can use to avoid purchase of canned black olives that contain unwanted amounts of acrylamide. Since the FDA data has shown no detectable levels of acrylamide in numerous samples of important olives packed in brine, those olives may be worth considering as options that may help avoid unwanted acrylamide. As stated previously, extra virgin olive oil is another form of this nutrient-rich food that, to our knowledge, has not been shown in research to contain unwanted amounts of acrylamide.
How does Food Freshness Affect Acrylamide Formation?
There is no direct research evidence relating food freshness to acrylamide formation. However, there is some interesting research relating the freshness of a food to the presence of asparagine in that food. Perhaps the most interesting case involves asparagus. Studies have shown that the amount of asparagine in asparagus can increase from 41 to 820 micromoles/gram (dry weight) over the course of post-harvest storage. Only 5 days of storage were required for those much higher levels of asparagine to be formed in the asparagus. Even though higher levels of acrylamide do not automatically form when asparagine is present in a food, and even though asparagus is not a red flag food when it comes to acrylamide, this relationship suggests that one of the building blocks for acrylamide—lasparagine—may be more limited when food is cooked in its freshest form. From our perspective, this information is just one more reason to think about fresh foods as our best bet when it comes to health.
How is Acrylamide Related to Human Metabolism?
Once ingested, acrylamide can be detoxified in the body if it is processed through our cytochrome P450 enzyme system and converted into glycidamide, or if it is hooked together with the sulfur-containing, antioxidant molecule called glutathione. Even though our metabolic pathways can help us detoxify acrylamide, however, we can still overload the detox capability of these pathways and put ourselves at health risk from excess exposure to this substance. The fact that we have detox capacity, however, makes it very likely that we can help lower our risk of problems from acrylamide if we have kept plenty of glutathione on hand in our metabolic reserves. One way to help support our glutathione supplies is to consume plenty of sulfur-containing foods (like onions, garlic, and cruciferous vegetables), and especially foods that contain significant amounts of the amino acid cysteine. (Cysteine is one of the key components of glutathione.) Cruciferous vegetables like broccoli and Brussels sprouts, onions, garlic, and red peppers are plant foods that can provide higher-than-average amounts of this amino acid. Poultry, yogurt, and eggs are animal foods that have good concentrations of cysteine.
WHFoods Recommendations
Your highest-risk foods for acrylamide exposure fall into three basic categories: (1) fried, processed foods like potato chips and french fries; (2) baked snack foods containing wheat and sugar, including cookies and crackers; and (3) processed foods involving toasted grains, including toasted wheat cereals, and roasted grain-based coffee substitutes. Roasted cocoa beans (and the chocolate made from them), some dehydrated soup mixes, and some canned black pitted olives can also fall into this higher-risk category in terms of acrylamide exposure.